Abstract
Background: Immunotherapy has become a major strategy for cancer therapy and has dramatically improved patients' outcomes, especially in hematologic malignancies, but little is known about the functional capacity of T cells in Acute Myeloid Leukemia (AML) patients. The AML Bone Marrow (BM) microenvironment is a dynamic structure that plays a crucial role in initiating and maintaining leukemogenesis and contributing to AML drug resistance and immune suppression, and it is likely that T cells extant in the AML environment are functionally impaired. For example, pre-clinical and clinical studies have identified the upregulation of PD-1/PD-L1 axis as a potential mechanism of resistance to T-cell therapy suggesting that a better understanding of the expression of immune regulatory molecules in AML will help guide future therapeutic interventions, as well as serve as potential predictive/prognostic biomarkers for T-cell therapies. Frequent bone marrow sampling is not feasible in AML patients and so it is important to understand whether peripheral blood T-cells reflect T-cell phenotypes in the BM, including expression of inhibitory molecules. The objective of this study is to evaluate the use of Peripheral Blood (PB) as a surrogate for BM to support periodic immunophenotype monitoring in a clinical trial setting.
Methods: Patient matched cryopreserved BM and PB primary AML samples (n=10 pairs) were surveyed by multiparameter flow cytometry to quantitate parameters including CD4 and CD8 effector T cell populations (Naïve: CD45RAhiCCR7hi, Central Memory: CD45RAloCCR7hi, Effector Memory: CD45RAloCCR7lo, Terminal Effector CD45RAhiCCR7lo), regulatory T cells (CD4+CD25+CD127-), expression of various immune checkpoint molecules (PD-1, TIM3, LAG3 and TIGIT), and leukemia cell phenotypes such as CD33, CD34, CD38, PD-1 and TIGIT ligands. All parameters collected for T cells and AML cells were analyzed using the Bland-Altman and Lin's concordance methods to evaluate the similarities between BM and PB. For eight samples, primary AML mononuclear cells (MNCs) were treated ex vivo for 4 days with Control or AMG 330 (CD33/CD3 BiTE®) and phenotyped to monitor response to BiTE® treatment and changes in checkpoint receptors.
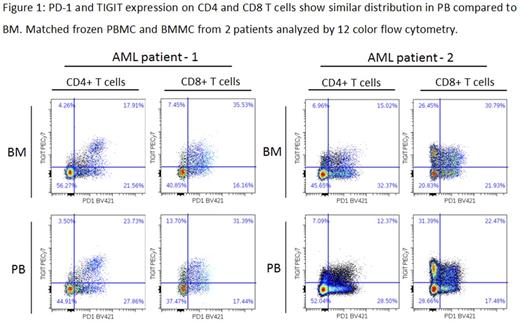
Results: PB is an appropriate surrogate for BM for many of the T cell and AML cell attributes analyzed. CD33 expression on blasts and the distribution of CD4 effector subsets in PB and BM were concordant based on Lin's concordance correlation, while CD4:CD8 ratio and T cell:blasts ratio were qualitatively similar (Spearman Rank). We also observed that expression of CD25 on CD4 and CD8 T cells and the AML progenitor population CD34+/CD38+ in PB and BM were linearly correlated. CD69 was consistently expressed on a higher percentage of CD4 and CD8 T cells in the BM compared to PB, consistent with its role in memory T cell retention in the BM. Checkpoint receptor expression was similar PB and BM (see Figure 1) but did not meet the statistical test for concordance; analysis of a larger number of samples will provide greater insights into non-correlated samples. We observed that most of the AML samples had a high median frequency of T cells expressing PD-1 (CD4+: 36%; CD8+: 46%) and TIGIT (CD4+: 22%; CD8+: 52%). TIM3 expression was lower (CD4+: 18%; CD8+: 17%) while LAG3 was rarely expressed on AML T cells (2/9 AML samples tested had >5% LAG3+). We also observed that PD-1 was expressed more frequently on CD8+ T cells from R/R AML BM compared to ND (medians: 43% vs 53%, respectively, p=0.022). TIGIT ligands, PVR and PVRL2, were present on AML blasts in the majority of patients (7/9 and 5/9 had >20% PVR+ or PVRL2+ respectively), while PD-L1 and PD-L2 were not expressed on blasts in any of the AML samples tested. To understand if these immunophenotypes relate to BiTE® responsiveness, primary AML MNCs (n=9) were treated ex vivo for 4 days with AMG 330. Response of these samples to treatment was dependent on CD33 expression and T cell:target cell ratio. Changes in PD-1 and TIGIT induced by ex vivo AMG 330 treatment will also be presented.
Conclusion: Using multiparameter flow analysis, we observed a strong correlation between BM and PB for several biomarkers tested including CD4:CD8 ratios, T cell effector subsets, and leukemia cell phenotypic markers. Our results suggest that PB immunophenotype is representative of the BM in AML and can be used in a clinical trial setting to gain relevant biomarker data.
Lesley: Amgen Inc.: Employment, Equity Ownership. Beutner: Amgen Inc.: Employment, Equity Ownership. Anderson: Amgen Inc.: Employment, Equity Ownership. Fitzpatrick: Amgen Inc.: Employment, Equity Ownership. Loberg: Amgen Inc.: Employment, Equity Ownership. Mehta: Amgen Inc.: Employment, Equity Ownership. Dos Santos: Amgen Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal